Various types of fuel are used as an energy carrier, for example, peat, coal, wood, as well as fuel briquettes. Coal is considered to be the most efficient type, allowing the boiler or furnace to work as efficiently as possible. In order to select a good fuel, several factors must be considered, including the temperature at which the coal burns.

When choosing a material, we must take into account several factors
Features of different types of fuel
Consider the two main, most common, types of solid fuel raw materials - firewood and coal.
Firewood contains a significant amount of moisture, so the moisture evaporates first, which requires a certain amount of energy. After the moisture evaporates, the wood begins to burn intensively, but, unfortunately, the process does not last long.
Therefore, in order to maintain it, it is necessary to regularly add firewood to the firebox. The ignition temperature of wood is about 300 ° C.
Coal surpasses wood in terms of the amount of heat generated and the duration of combustion.... Depending on the age of the fossil material, the mineral is divided into types:
- brown;
- stone;
- anthracite.
With the help of technical analysis, ash content, moisture, sulfur and phosphorus content, the release of volatile substances on the combustible mass, the heat of combustion and the characteristics of the non-volatile solid residue are determined in coals and oil shale. All analyzes are carried out on the basis of analytical samples of coal and shale, and the moisture content in the working fuel - on the basis of laboratory samples.
The recalculation of the elemental composition, the yield of volatile substances and the heat of combustion for coals (except for shale) during the transition to another mass is carried out according to the ratios, according to the formulas. When recalculating the elemental composition and calorific value of shale, the ash content A must be replaced by A + CO2 for the corresponding mass of shale.
MOISTURE
When analyzing coals, the following types of moisture are distinguished:
- laboratory - Wl, determined by laboratory samples for technical analyzes;
- analytical - Wа, determined by analytical samples for elementary analysis;
- air-dry - Wavs, determined by analytical samples in the air-dry state of the sample under the conditions of the actual state of air in the laboratory by relative humidity and temperature;
- hygroscopic (internal) - Wgi, close to Wa, but determined by analytical samples brought to an air-dry equilibrium state at * constant relative humidity (60 ± 2%) and air temperature (20 ± 5 ° C);
- working moisture - Wp determined from a laboratory sample, taking into account the loss of moisture when the sample is sent to the laboratory.
Working fuel moisture is subdivided into internal moisture, equal to hygroscopic (Wdi), and external moisture (Wout), defined as the difference Wout = Wp-Wg,%. Internal hygroscopic moisture (Wdi) depends on the relative humidity and temperature of the ambient air and the adsorption capacity of the coal. Moisture and ash content that make up the ballast Br = Wp + Ap of the fuel, in particular external moisture, deteriorate the quality of coals, reduce flowability, complicate classification and transportation, and cause coal freezing in winter.
Coals with a high moisture content are unsuitable for long-term storage, since moisture promotes self-heating and spontaneous combustion. In connection with these technical conditions and standards for coals by type of consumption, limit (rejection) standards for moisture content have been established for certain grades and grades of coal.
Lean coals, semi-anthracite and anthracite are less moist, brown coals are more moist. The moisture content in coals and oil shale is determined in accordance with GOST 11014-2001. The essence of the method for determining the moisture content is to dry a fuel sample in an oven at a temperature of 105-110 ° C to constant weight and to calculate the weight loss of the sample taken in percent. Determination of moisture content by an accelerated method is carried out according to GOST 11014-2001 The essence of the accelerated method for determining the moisture content consists in drying a sample of fuel in an oven at a temperature that rises within 5 minutes from 130 to 150 ° С for an analytical sample and within 20 minutes for a laboratory sample, and in calculating the weight loss of a sample of fuel taken as a percentage ... Discrepancies between the results of two parallel determinations of moisture content according to the specified GOST should not exceed the permissible values.
ASH


Coals always contain incombustible mineral impurities, which include calcium carbonates CaCO3, magnesium MgCO3, gypsum CaS04-2H20, pyrite FeS2, and rare elements. When coal is burned, the unburned part of the mineral impurities forms ash, which, depending on its composition, can be refractory or low-melting, free-flowing or fused. Mineral impurities deteriorate the quality of coals, reduce the heat of combustion, load transport with excess ballast, increase the consumption of coal per unit of output, complicate the conditions of use and worsen the quality of coke.
Mineral impurities are not always ballast, sometimes they contain rare elements in quantities that allow their industrial use. In addition, slag can be used to make cement and other building materials.
The ash content of coals is determined according to GOST 11022-95. The essence of the method consists in ashing a sample of fuel in a muffle and calcining the ash residue to a constant mass at a temperature of 800-825 ° C for coals and 850-875 ° C for oil shale and determining the mass of the ash residue as a percentage of the mass of the fuel sample. The ash content obtained as a result of the analysis of the analytical sample is recalculated for the ash content in absolutely dry fuel Ac.
The ash content of the working fuel Ap in percent is calculated by the formula:
Ap = Ac (100-Wp) / 100
Determination of ash content by an accelerated method is carried out in accordance with GOST 11022-95. Its essence lies in ashing a sample of coal in a muffle heated to a temperature of 850-875 ± 25 ° C, and determining the mass of the ash residue as a percentage of the mass of the sample.
Discrepancies between the results of determining the ash content of Ls based on duplicates of one laboratory sample in different laboratories according to the specified GOSTs should not exceed:
for fuels with ash content:
- up to 12% ... 0.3%
- from 12 to 25% ... 0.5%
- over 25% ... 0.7%
- over 40% ... 1.0%
The technical conditions and GOSTs establish average and maximum (rejection) norms of ash content for various grades and classes of coal for individual mines, open-pit mines and processing plants.
SULFUR
The total sulfur contained in coals consists of pyrite Sc, sulfate Sc, and organic Sо sulfur. Pyrite sulfur occurs in coals in the form of individual grains and large pieces of pyrite and marcasite minerals. When coal is weathered in mines, open pits and on the surface, pyrite oxidizes and forms sulfates. Sulphate sulfur is contained in coals, mainly in the form of iron sulphates FeSO4 and calcium CaSO4. The content of sulfate sulfur in coals usually does not exceed 0.1-0.2%. When burned, sulphate sulfur turns into ash, and when coal is coked, it turns into coke. Organic sulfur is part of the organic matter of coal. The content of total sulfur and its variety in the fuel is determined in accordance with GOST 8606-93.
Sulfur is found in all types of solid fuels, and the total sulfur content in coals ranges mainly from 0.2 to 10%.
Sulfur is an unwanted and even harmful part of the fuel. When coal is burned, it is released in the form of SO2, polluting and poisoning the environment and corroding metal surfaces, reduces the heat of combustion of fuels, and during coking it passes over, deteriorating its properties and the quality of the metal. The choice of ways of using coals often depends on their total sulfur content. That is why total sulfur is the most important indicator of coal quality.
The total sulfur content is determined by burning a sample of fuel with a mixture of magnesium oxide and sodium carbonate (Eshch's mixture), dissolving the formed sulfates, precipitating the sulfate ion in the form of barium sulfate, determining the mass of the latter and recalculating it to the mass of sulfur. The sulfate sulfur content is determined by dissolving the sulfates contained in the fuel in distilled water, precipitating the sulfate ion in the form of barium sulfate, determining the mass of the latter and recalculating it to the mass of sulfur. The content of pyrite sulfur is determined by processing a fuel sample with dilute nitric acid and dissolving sulfates in it, formed during the oxidation of pyrite with nitric acid, followed by precipitation of the sulfate ion in the form of barium sulfate, determining the mass of the latter and recalculating it to the mass of sulfur. The content of pyrite sulfur is determined by the difference between the content of sulfur recovered from the fuel by nitric acid and water.
The discrepancy between the results of two parallel determinations of sulfur content in one laboratory should not exceed: for coal with a sulfur content of up to 2% - 0.05%, over 2% - 0.1%. The discrepancies between the results of determining the sulfur content from duplicates of one laboratory sample in different laboratories should not exceed: for coal with a sulfur content of up to 2% - 0.1%, over 2% - 0.2%. The sulfur content is determined by the accelerated method according to GOST 2059-54.
The essence of this method consists in burning a bulk of coal in a stream of oxygen or air at a temperature of 1150 ± 50 ° C, trapping the formed sulfur compounds with a solution of hydrogen peroxide and determining the volume of sulfuric acid obtained in a solution by titrating it with a solution of caustic potassium. The discrepancy between the results of two parallel determinations of the sulfur content of one sample for one laboratory should not exceed 0.1%, for different laboratories - 0.2%.
PHOSPHORUS
It is contained in coal in insignificant amounts - 0.003-0.05% and is a harmful impurity, since during coking it turns into coke, and from coke - into metal, imparting brittleness to it. In Donetsk coals, the phosphorus content ranges from 0.003 to 0.04%, in Kuznetsk and Karaganda - 0.01 to 0.05%. Phosphorus is determined by volumetric or photocolorimetric method according to GOST 1932-93.
The volumetric method consists in the oxidation of phosphorus contained in a coal sample into orthophosphoric acid, followed by the precipitation of phosphorus in the form of phosphoric-libdicate ammonium, dissolving the latter in an excess of a titrated solution of caustic alkali, back titrating the resulting solution with sulfuric acid and calculating the percentage of phosphorus by the amount of alkali solution consumed to dissolve the precipitate. The photocolorimetric method consists in burning a sample of coal with a mixture of magnesium oxide and sodium carbonate (Eshch mixture), dissolving the caked mass in acid, removing silicic acid from the solution, and photocolorimetric determination of phosphorus in the filtrate.
The discrepancy between the results of two parallel determinations of the phosphorus content should not exceed:
With phosphorus content:
- up to 0.01% ... 0.001%
- up to 0.05% ... 0.003%
- up to 0.1% ... 0.005%
- more than 0.1% ... 0.01%
Calculation of the phosphorus content is carried out on an absolutely dry mass of coal.
VOLATILES
When coals are heated without air access, solid and gaseous products are formed. The release of volatile substances is one of the main indicators for the classification of coals by grades and depends on the degree of coal metamorphism.With the transition to more metamorphosed coals, the yield of volatiles decreases. Thus, the yield of volatile substances per combustible mass Vg for brown coals ranges from 28 to 67%, for bituminous coals - from 8 to 55% and for anthracite - from 2 to 9%. The yield of volatile substances for bituminous and brown coals is determined according to GOST 6382-65 by the weight method, and for anthracite and semi-anthracite of the Donetsk basin - according to GOST 7303-2001 according to the weight method, and for anthracite and semi-anthracite of the Donetsk basin - according to GOST 7303-90 by the volumetric method.
The essence of the gravimetric method consists in heating a sample of coal in a lidded porcelain crucible at a temperature of 850 ± 25 ° С for 7 min and determining the weight loss of the sample taken. The volatiles yield is calculated from the difference between the total mass loss and the loss due to moisture evaporation and the removal of carbon dioxide from carbonates when the latter content in the sample is more than 2%. Discrepancies between the results of determining the yield of volatile substances Vg should not exceed 0.5% for coals with Vg less than 45% and 1.0% for coals with Vg> 45%.
The essence of the volumetric method consists in heating a sample of anthracite and semi-anthracite at a temperature of 900 ± 10 ° C for 15 min and determining the volume of the evolved gas in cm3 / g. The discrepancy between the results of two parallel determinations of the volumetric yield of volatile substances in cm3 / g for one sample should not exceed 7% to the smaller of them.
Based on the values of the yield of volatile substances and the characteristics of the non-volatile residue, it is possible to roughly estimate the caking capacity of coals, as well as predict the behavior of the fuel in the technological processes of processing and propose rational combustion methods.
COMBUSTION HEAT
Heat of combustion (Q, kcal / kg) is one of the main indicators of coal quality. The standards and specifications provide for the average value of the heat of combustion of fuel per combustible mass for a bomb Qgb for coal, and for shale for absolutely dry fuel - Qsb. The heat of combustion is determined according to GOST 147-95.
The essence of the method consists in burning a sample of fuel in a calorimetric bomb in compressed oxygen and determining the amount of heat released during its combustion. The heat of combustion per combustible mass Qgb, determined from the bomb, contains, in addition to the heat obtained from the combustion of the combustible part of coal, the heat released during the formation and dissolution of nitric acid in water, and the latent heat of vaporization during the combustion of hydrogen, which is transferred to the calorimeter water. The lowest calorific value Qgn is obtained as the difference between Qgb and the heat obtained in the bomb due to acid formation and condensation of water vapor, which in practical conditions of coal combustion cannot be used.
The lowest calorific value Qgn is obtained as the difference between Qgb and the heat obtained in the bomb due to acid formation and condensation of water vapor, which in practical conditions of coal combustion cannot be used:
Qгн = Qgb - 22.5 (Sro + Srk) - aQgb - 54Ng, where 22.5 is the heat released during the formation of sulfuric acid in water by 1% of sulfur, which is converted into sulfurous acid when burning coal in a bomb, kcal; Sro + Srk - the amount of combustible sulfur, which was converted during the combustion of coal in a bomb into sulfurous acid (in percent), referred to the combustible mass of the coal sample.
The lowest heat of combustion of coal per working mass Qрн, released during the combustion of fuel in industrial furnaces, is lower than Qгн, since the working fuel contains ballast Br = Wр + Aр and, in addition, to evaporate moisture, it is required to spend 6Wr heat;
Qрн for coals can be calculated by the formula:
Qрн = Qгн100 - Wp - Ap100 - 6Wp, kcal / kg,
where Qрн is the lowest combustion heat per working mass, kcal / kg; Qgn is the lowest heat of combustion per combustible mass, kcal / kg.
For oil shale Qрн - is calculated by the formula
Qрн = Qгн100 - Wp - Wpcap - COp2K100 - 6Wp - 9.7COp2K,
where 9.7COp2K - heat absorption during decomposition of carbonates contained in shale, kcal / kg.
CONDITIONAL FUEL
Due to the fact that the heat of combustion of coals of individual deposits, grades and grades and other types of fuel is different, for the convenience of planning fuel needs, determining specific rates and actual fuel consumption, as well as for the possibility of their comparison, the concept of "conventional fuel" has been introduced. Such fuel is taken as conditional, the lower heat of combustion of which for the working mass Qрн is 7000 kcal / kg. To convert natural fuel into conditional and conditional into natural fuel, calorie equivalent is used, the value of which depends on Qрн.
CALORIE EQUIVALENT
The caloric equivalent EK is the ratio of the lowest calorific value of the working fuel to the calorific value of the standard fuel, i.e.
Ec = Qрн7000.
The conversion of natural fuel Vn into conditional Vu is made by multiplying the amount of natural fuel by the calorie equivalent: Vu = Vn * Eq.
Conversion of the equivalent fuel into natural fuel is done by dividing the amount of the equivalent fuel by the calorie equivalent: Vu = Vn / Eq.
TECHNICAL EQUIVALENT
The technical equivalent is used to compare different coals and other fuels in terms of their thermal value and to determine the equivalent amounts when replacing one type of fuel with another. The technical equivalent Et is the ratio of the useful amount of heat of the given fuel to the heat of combustion of the standard fuel. Usefully used heat per unit mass of fuel is expressed by the product of the lowest heat of combustion of the working fuel Qрн by the efficiency of the installation. Thus, the technical equivalent, in contrast to the high-calorie one, takes into account not only the value of the heat of combustion of a given fuel, but also the degree of possible heat engineering use, is determined by the formula:
Et = QrnYk7000,
where Yk is the efficiency of this boiler plant in unit fractions; 7000 is the heat of combustion of the equivalent fuel, kcal / kg.
The technical equivalent for the same fuel is always less than the calorie equivalent. The technical equivalent is practically used in determining the specific rates and actual fuel consumption.
Fuel composition of different types
Brown coal belongs to young deposits, therefore it contains the greatest amount of moisture (from 20% to 40%), volatile substances (up to 50%) and a small amount of carbon (from 50% to 70%). Its combustion temperature is higher than that of wood, and is 350 ° C. Calorific value - 3500 kcal / kg.
The most common type of fuel is bituminous coal. It contains a small amount of moisture (13-15%), and the content of the fuel element carbon exceeds 75%, depending on the grade.
The average ignition temperature is 470 ° C. Fugitive gases in coal 40%. During combustion, 7000 kcal / kg are released.
Anthracite, which occurs at a considerable depth, is among the oldest deposits of solid-fuel fossil. It contains practically no volatile gases (5-10%), and the amount of carbon varies between 93-97%. The heat of combustion is in the range from 8100 to 8350 kcal / kg.
Charcoal should be noted separately. It is obtained from wood by pyrolysis - combustion at high temperatures without oxygen. The finished product has a high carbon content (70% to 90%). When wood fuel is burned, about 7000 kcal / kg are emitted.
You can read about the features of using peat briquettes in this article:
Thermal characteristics of wood
Charcoal is classified as a separate category as it is not a fossil fuel, but a product of production. To obtain it, wood is treated in a special way in order to change its structure and remove excess moisture.The technology of obtaining an efficient and easy-to-use energy carrier has been known for a long time - before, wood was burned in deep pits, blocking the access of oxygen, but today special charcoal kilns are used.


Burning wood in a charcoal kiln
Under normal storage conditions, the moisture content of charcoal is about 15%. Fuel ignites already when heated to 200 ° C. The specific calorific value of the energy carrier is high - it reaches 7400 kcal / kg.
The combustion temperature of charcoal varies depending on the type of wood and combustion conditions. For example, birch coals can be used to heat up a forge and forge metal - with intensive air supply, they will burn at 1200-1300 ° C. In a stove or heating boiler, the temperature during combustion will reach 800-900 ° C, and when using coal in the grill on the street - 700 ° C.
Burned wood fuel is economical - its consumption is much lower compared to using firewood. In addition to high heat transfer, it is characterized by low ash content.
Due to the fact that charcoal burns with a small amount of ash and gives off an even heat without an open flame, it is ideal for cooking meat and other foods over an open fire. It can also be used for fireplace heating or cooking on a cooking stove.
Wood species differ in density, structure, quantity and composition of resins. All these factors affect the calorific value of the wood, the temperature at which it burns, and the characteristics of the flame.
Poplar wood is porous, such firewood burns brightly, but the maximum temperature indicator reaches only 500 degrees. Dense wood species (beech, ash, hornbeam), when burned, emit over 1000 degrees of heat. Indicators of birch are slightly lower - about 800 degrees. Larch and oak flare up hotter, giving out up to 900 degrees Celsius. Pine and spruce firewood burns at 620-630 degrees.
Birch firewood has a better ratio of heat efficiency and cost - it is economically unprofitable to heat with more expensive woods with high combustion temperatures.
Spruce, fir and pine are suitable for making fires - these conifers provide relatively moderate warmth. But it is not recommended to use such firewood in a solid fuel boiler, in a stove or fireplace - they do not emit enough heat to effectively heat the home and cook food, burn out with the formation of a large amount of soot.
Low-quality firewood is considered to be fuel made from aspen, linden, poplar, willow and alder - porous wood emits little heat when burning. Alder and some other types of wood "shoot" coals during combustion, which can lead to a fire if the wood is used to fire an open fireplace.
When choosing, you should also pay attention to the degree of moisture content of the wood - raw firewood burns worse and leaves more ash.
Currently, there is a tendency to switch from installations, which were based on the process of gas combustion, to solid fuel heating domestic systems.
Not everyone knows that the creation of a comfortable microclimate in the house directly depends on the quality of the selected fuel. We will single out wood as a traditional material used in such heating boilers.
In harsh climatic conditions, characterized by long and cold winters, it is quite difficult to heat a house with wood for the entire heating season. With a sharp drop in air temperature, the owner of the boiler is forced to use it on the verge of maximum capabilities.
When choosing wood as a solid fuel, serious problems and inconveniences arise. First of all, we note that the combustion temperature of coal is much higher than that of wood.Among the disadvantages is the high speed of combustion of firewood, which creates serious difficulties in the operation of the heating boiler. Its owner is forced to constantly monitor the availability of firewood in the firebox; a sufficiently large amount of them will be required for the heating season.
Combustion process
Depending on the type and grade, the fuel is divided into short-flame and long-flame. The short-flame ones include anthracite and coke, charcoal.
When burned, anthracite generates a lot of heat, but to ignite it, you need to provide a high temperature with a more flammable fuel, for example, wood. Anthracite does not emit smoke, burns odorlessly, its flame is low.
Long-flame fuels are burned in two stages. First, volatile gases are released, which are burned above the coal layer in the furnace space.
After the gases are burned out, the remaining fuel begins to burn, which in the meantime has turned into coke. Coke burns with a short flame on the grates. After carbon burnout, ash and slag remain.
Natural fuel stove properties
It is the cheapest way to make a brick heating stove on coal with your own hands.
Materials (edit)
We need:
- brick;
- ready-mixed mortar for laying ovens;
- cast iron grate;
- cast iron cooking stove;
- metal sheet b = 4mm - 600x1200 mm - 0.72 m2;
- welding electrodes - 1 pack.
Instruments
- trowel;
- trowels;
- hammers;
- drill;
- other.
Scheme and order
Photo №1 General view
Photo # 2 Poryadovka
Description of masonry
- On top, without mortar, put a brick (see photo # 2, first row). We strictly control horizontality using a level.
- Install the blower door. We fix it with a wire and wrap it with an asbestos cord.
- We put grates directly above the blower.
- We continue laying in accordance with the order (see photo No. 2)
- Install the firebox door. We fix it with wire and bricks.
- From above, the row should overlap the fire door and end 130 mm above it.
- We continue laying, slightly shifting the bricks back. Before that, we lay an asbestos cord, on which we will install the hob.
- Let's start the formation of the chimney from the next row. The design provides for the installation of a shell pipe made of sheet metal or corrugated aluminum. The pipe should not be heavy. Otherwise, the center of gravity may shift.
- On the eleventh row, we put a valve to regulate the air flow. Do not forget to seal it with an asbestos cord and cover it with clay.
- Next, we put the chimney in the quadruple, which we join with the metal one. The pipe should be strictly vertical and not bend to the side. For greater stability, it should be covered with three rows of bricks.
- We remove the knockout bricks that we put on the 4th row, we clean the chimney from debris.
- Now the coal stove should be whitewashed. Any lime will go. Experts recommend adding blue and a little milk. So the whitewash will not darken and fly off.
- We install a metal sheet in front of the firebox.
- Install the skirting board
Do-it-yourself coal stove is not easy. It is better to seek help from an experienced stove-maker or be patient.
The design of a coal stove is not much different from a wood-burning device, but there are some features. The principle of air supply required for combustion is significantly different. In coal stoves, it must come from the bottom to provide airflow to the fuel, and in wood-fired air intake systems they are located above
Coal-fired devices are less demanding on fuel: it is important that the primary kindling is carried out with dry material; during the heating process, the dryness of the fuel is desirable, but not essential. Before use, coal is recommended to be heated in a specially designed compartment of the furnace.
The flue system for a coal stove is equipped so that the flow of air with combustion products moves intensively through the pipe.The flow rate is regulated not with the help of a damper view (it may not exist at all), but with a blower. All these design features are due to the duration of the fuel burnout.
Coal furnace chimney design
High performance. If the chimney system is built correctly, a coal stove will become an efficient and reliable heating system for your home. It can also be a good backup or add-on option.
Multifunctionality. There are industrial models designed not only for heating, but also for cooking, heating water. Homemade brick and metal ovens are also often made with a hob and / or built-in bins.
Fuel availability. There are areas where coal is readily available and relatively cheap. For such settlements, coal heating is economically profitable.
Simple construction. A conventional solid fuel stove does not require mechanical attachments. There are no electromechanical structural elements in it that can break at the most inopportune moment. True, this does not apply to complex modern models with automatic fuel supply.
Possibility to heat with wood. In practice, devices that run exclusively on coal are almost never found on the market. The stoves can be fired with both coal and wood. Also, manufacturers of heating equipment manufacture combined heat generators capable of operating on gas and solid fuels.
We offer you to familiarize yourself with Interior design in the relaxation room in the bath
Industrial coal furnace
Fire hazard. Any heating equipment that uses wood or coal is potentially dangerous. During installation, you should strictly adhere to the rules and regulations stipulated by SNiP 2.04.05-91.
Fuel storage required. Usually, coal is purchased before the start of the heating season; a separate room should be allocated for its storage.
You have to constantly monitor the operation of the oven. If the home owner installs a conventional stove, and not a model with an automatic fuel supply, then he needs to constantly add coal to the firebox and monitor its operation.
Uneven heating of the house. To ensure that all rooms are well heated, it is necessary to provide a system for the distribution of thermal air. Otherwise, the room where the stove is installed will be heated too hot, and the rest of the rooms will be noticeably cooler.
Chimney cleaning. Solid fuel stoves require constant care, regular inspection and maintenance.
Environmental pollution. Combustion of solid fuels is more harmful to the environment than heating with liquid or gaseous fuels. This has led to some restrictions on the use of coal-fired stoves, which may be imposed by local authorities in some regions.
Coal boiler device for home heating
Foundation for a brick oven.
As already mentioned, the combustion temperature of coal is quite high. With sufficient air flow into the firebox, it reaches 1000-1100 ° C, so not every material is able to withstand such conditions for a long time.
For comparison: dry wood under identical circumstances is capable of giving no more than 700 ° C in the firebox, and even then very rarely. In addition, coal fuel is much more nutritious than firewood.
| Type of fuel | Calorific value | |
| MJ / kg | kW / kg | |
| Wood moisture 25% | 10,1 | 2,8 |
| Hard coals | 21,5 | 5,9 |
| Brown coals | 15,5 | 4,3 |
Previously, in old houses, heating stoves or stoves were laid out only of solid red brick. With constant burning of high-calorific coal from the high temperature, the masonry began to crumble, so the owners lined the firebox from the inside with thick steel soles from the railway tracks to protect the walls.
At the moment, the problem of coal combustion is solved much easier - with the help of fireclay bricks. The design of the furnace provides for lining the fuel chamber with fireclay stone of the SHA, SHB or SHV grade to a thickness of a quarter or half a brick. This material is able to maintain a temperature of 1400 ° C without problems and for a short time - up to 1650 ° C.
Furnace masonry tools.
There is another point: due to the higher calorific value than wood, a greater amount of heat is released, part of which goes with the combustion products into the chimney.
To avoid this, a more developed network of smoke circuits is provided in the coal furnace, where the flue gases have time to transfer heat to the brick walls, and not fly out straight into the chimney.
Otherwise, this is an ordinary brick stove with all the advantages and disadvantages.
The most popular and demanded manufacturers of coal stoves on the market are Spanish (Josper S.A.) and Movilfrit. The features and advantages of these coal-fired furnaces are discussed below.
The manufacturer of coal stoves "Josper" has managed to gain a leading position in the production of stoves that use wood fuel. The closed grill-ovens of this company perfectly cope with the load in a catering establishment with a number of seats from 30 to 100. Mobile coal ovens are in the greatest demand, the design of which has:
- pedestal for coal or firewood;
- ash pan;
- a closed shelf for temporary storage of food in a hot state;
- exhaust umbrella.
The owner of the establishment should be attracted by the fact that the use of Josper stoves will make it possible to reduce fuel consumption. Compared to classic barbecue systems, the savings in coal exceed 25%, which makes it possible to recoup the cost of a coal stove in a short period of time. Practice confirms that the price of coal stoves is fully justified.
The manufacturer is allowed to use wood charcoal or vegetable charcoal for cooking. Food is cooked directly on the wire racks, while cooking on two wire racks is allowed. The Josper charcoal stoves are practically the only ones in which a charcoal stove and a charcoal barbecue are combined. The dishes prepared with this equipment are very tasty and aromatic.
fat does not get on the coals, but when the grate is tilted, it flows into a special cell, which is cleaned as it is filled. Also, all the grates have special hooks, which makes it possible to change the grates while hot. Ashes are automatically fed into a special hopper that slides out for cleaning.
- chicken thighs will cook in 3 minutes;
- beef steaks in 6 minutes,
- and the potatoes will bake for 10 minutes.
This fast cooking time is ensured by the high operating temperatures.
Burning
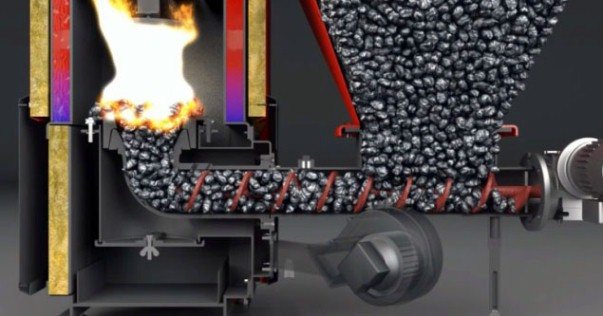
Consider the process of burning fuel in a conventional stove, which is used to heat private houses. It consists of the main parts:
- firebox;
- blower;
- chimney with a pipe.
The firebox is connected to the blower through a special grate (grate) located at the bottom of the firebox... Fuel is placed on the grate, and air from the blower through the grate enters the firebox.
On burning coal in furnaces
The above temperatures in degrees for each type of fuel are theoretical. That is, they are achievable under ideal conditions for the combustion of an energy carrier, which does not happen in real life, and even at home. Moreover, it makes no sense to overheat a brick stove or a metal boiler. They are not designed for such regimes.


By and large, the intensity of coal combustion in the stove depends on the amount of air supplied. Coals give off heat best with 100% air supply, but in practice this does not happen, since we limit the amount of it with a damper or damper. Otherwise, the temperature in the combustion chamber will rise too much, and so it is in the range of 800-900 ºС.
As for a solid fuel boiler, an excessively intense combustion mode can cause a rapid boiling of the coolant and a subsequent explosion. Therefore, this type of solid fuel is burned in boilers in two ways:
- traditional, with loading into the furnace and limiting the amount of air.
- with the help of a metered feed, implemented in automatic boilers.

Combustion formulas


Ignition temperatures of different fuels (click to enlarge)
When fuel (wood, coal) ignites, a chemical reaction takes place with the release of heat.
Carbon dioxide reacts with the carbon in the fuel in the upper layers to form carbon monoxide.
This is not the end of the combustion process, because as it rises up in the furnace space, carbon monoxide reacts with oxygen from the air, the inflow of which occurs through the blower or the open door of the furnace.
Its combustion is accompanied by a blue flame and heat release. The resulting carbon monoxide (carbon dioxide) enters the chimney and escapes through the chimney.
Smoldering with minimal oxygen supply will result in the formation of non-toxic carbon monoxide, giving even heat.
Application
The main use of fuel is combustion to generate heat. Heat is used not only for heating a private house and cooking, but also in industry to support technological processes that take place at high temperatures.
Unlike a conventional stove, where the oxygen supply process and the combustion intensity are poorly regulated, in industrial furnaces, special attention is paid to controlling the oxygen supply and maintaining a uniform combustion temperature.
Let's consider the basic scheme of coal combustion.
- Fuel heating and moisture evaporation is in progress.
- As the temperature rises, the coking process begins with the release of volatile coke oven gases. Burning out, it gives the main heat.
- The coal turns into coke.
- The combustion process of coke is accompanied by the release of heat sufficient to start coking the next portion of the fuel.
In industrial boilers, the combustion of coke is separated into different chambers from the combustion of coke oven gas. This allows for the inflow of oxygen for coke and gas with different intensities, achieving the required burning rate and maintaining the required temperature.
Maximum combustion temperature of coal (video)
Today, this use of a variety of solid fuels, in the form of wood, coal or peat, is popular. It is used not only in everyday life for heating or cooking, but in many industries.
For homeowners who use various types of solid fuels to heat their homes, such a parameter as the burning temperature of coal is of considerable interest. Logically speaking, the higher this temperature, the more heat can be obtained by burning fuel. But this is theory, but in practice everything happens a little differently. The real burning of this valuable fossil will be discussed in this material.
Using charcoal
Charcoal is used in everyday life for cooking meat on the grill.
Due to the high combustion temperature (about 700 ° C) and the absence of flame, a uniform heat is provided, sufficient for cooking meat without charring.
It is also used as fuel for fireplaces, cooking on small stoves.
In industry, it is used as a reducing agent in metal production. Irreplaceable charcoal in the production of glass, plastics, aluminum.
It is possible to make charcoal yourself. Details:
Which charcoal is best for kebabs
Birch
"It was better to take a birch." Do you often hear such words while frying kebabs? Interestingly, the authors of these words cannot explain why. Just birch, gives the most suitable temperature. It is used not only for barbecue, but also in ovens.
Be careful: in summer you can buy ready-made coal in packages, but often under the guise of birch coal, they sell pine coal.
How to recognize birch charcoal
- anthracite color; - glossy twist; - the surface sparkles;
Pine coals have absolutely no luster and are painted in a simply rich, black color.
Briquettes


It is also recommended to use them for barbecues. At its core, it is also coal, only tightly pressed. The briquette is twice as dense. Than ordinary coal and burns much longer, reaching a temperature of 700 C. Also, they emit less smoke.
Oak
Such coal is rarely found in bags, but it is. It keeps the temperature for a long time, but it is quite difficult to kindle it. Therefore, it is mainly used in cafes and restaurants.
Pine
Poor quality, as indicated by its low price. On packages with such coal, they often write simply - "charcoal". Burns quickly and often smokes.




















